परिगणनात्मक नैनो-विज्ञान
परिगणनात्मक नैनो-विज्ञान
Our group uses the techniques of density functional theory to understand why the properties of matter change at the nanoscale. This understanding is then leveraged to design novel nanomaterials designed for specific applications.
Density functional theory: This is a very powerful computational technique for solving the quantum many body problem 'ab initio' or from 'first principles'. The only empirical input to the calculation is atomic numbers and atomic masses. For developing this technique, Walter Kohn was given the Nobel Prize in 1998.
Matter at the nanoscale: When one or more dimensions of a material have nanoscale dimensions, novel properties emerge, that are different from those of the corresponding bulk material. Electronic, magnetic, chemical and magnetic properties can change. These changes can be exploited to make better devices, catalysts or magnets.
Here are just a few examples of work done in our group, often with experimental collaboration.
1) METAL NANOPARTICLES
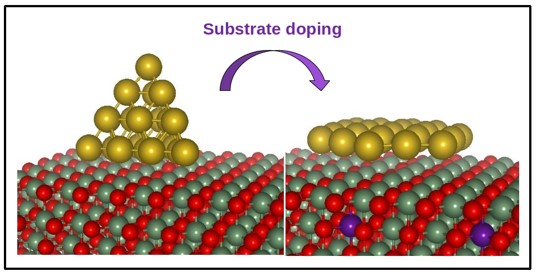
- Controlling the shape of nanoparticles: Many important catalysts consist of metal nanoparticles deposited on oxide supports. These nanoparticles usually clump up into a three-dimensional shape. We predicted that one can instead get a flat planar shape by doping the oxide support with an electron donor. Subsequent experiments in the group of H.J. Freund at the FHI, Berlin, confirmed our prediction. We also predict that these flat nanoparticles will be better catalysts.
- Nisha Mammen, Shobhana Narasimhan and Stefano de Gironcoli, J. Am. Chem. Soc. 133, 2801 - 2802 (2011).
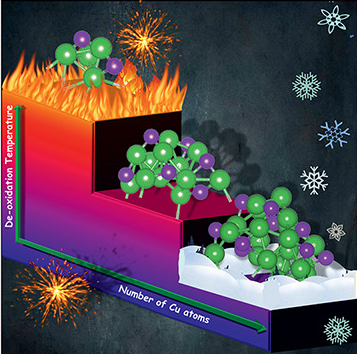
- Controlling the oxidation of nanoparticles: We show that by suitable choice of an oxide support, one can reverse trends in the size-dependence of the temperature at which metal nanoparticles transition from being oxidized to elemental. This is important in designing their use as nanocatalysts. This work was done in collaboration with the experimental group of S. Vajda at Argonne National Laboratory.
- N. Mammen, L. Spanu, E. Tyo, B. Yang, A. Halder, S. Seifert, M. J. Pellin, S. Vajda and S. Narasimhan, European Journal of Inorganic Chemistry, 16 - 22 (2018).
- Understanding the diffusion and sintering of supported nanoparticles. One of the major causes of degradation of supported metal nanocatalysts is sintering: at elevated temperatures, the nanoparticles diffuse and coalesce. We have studied this process, showing a scaling behavior that, surprisingly, depends on the melting temperature of the bulk metal. Depending on the metal, sintering occurs either by monomer addition or particle migration and coalescence.
- Nisha Mammen and Shobhana Narasimhan, J. Chem. Phys.151, 144709 (2019).
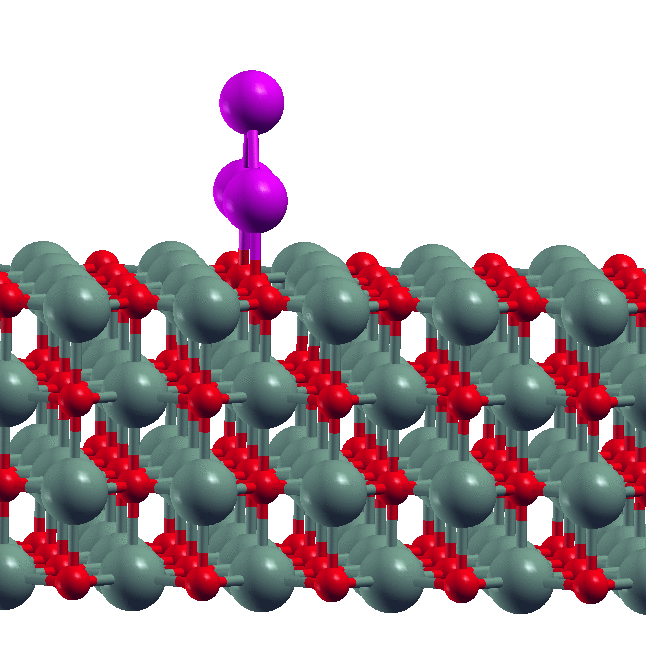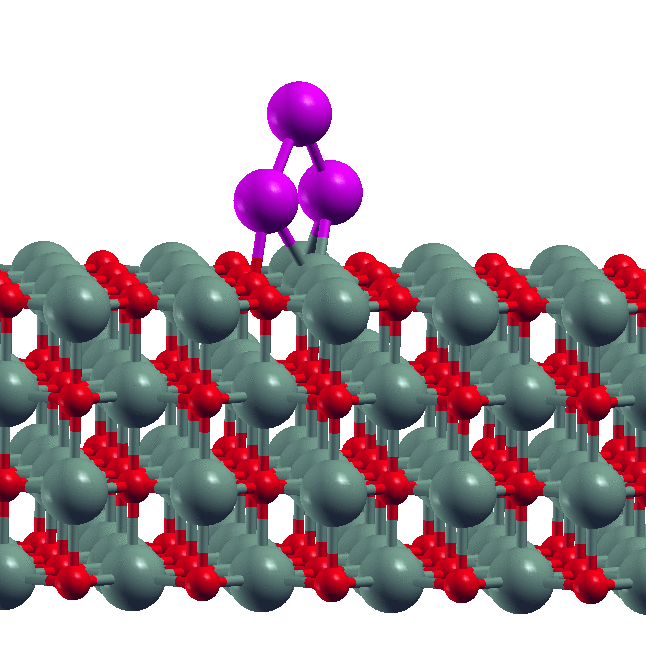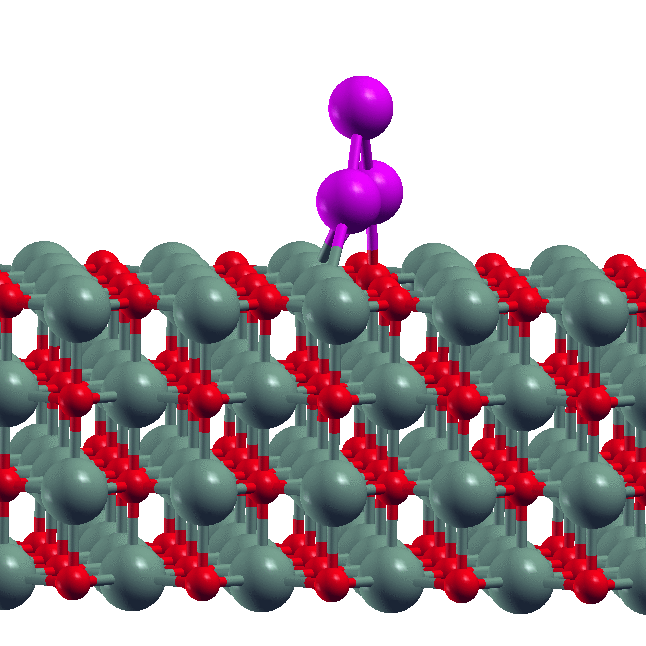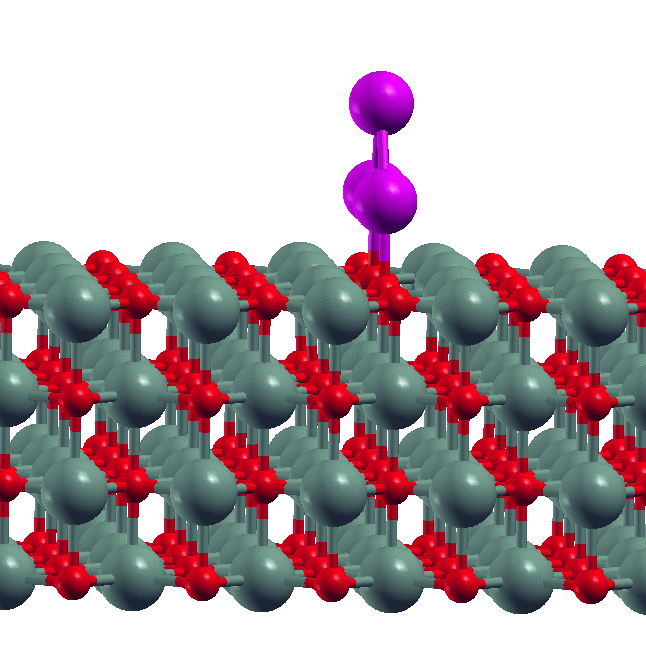
- Size-dependent dissolution of Pt nanoparticles Though sintering is a commonly observed phenomenon for metal nanoparticles, our experimental collaborators in the group of S. Goswami (IACS, Kolkata) found the reverse phenomenon of dissolution of Pt nanoparticles in the presence of certain organic ligands. Our DFT calculations showed why this happens. We derived a simple formula relating the maximum of size of nanoparticle that is dissolved to the metal-ligand binding energy.
- D. Sengupta, S. Goswami, R. Banerjee, M.J. Guberman-Pfeffer, A. Patra, A. Dutta, R. Pramanick, S. Narasimhan, N. Pradhan, V. Batista, T. Venkatesan and S. Goswami, Chemical Science, 11, 9226 - 9236 (2020).
2) MOLECULES ON SURFACES
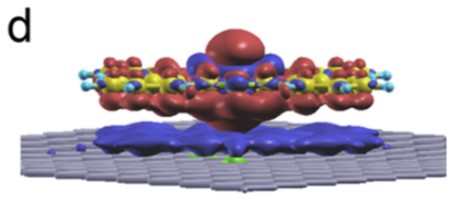
- Controlling the charge of metal centers in molecular adsorbates. Cobalt phthalocyanine is an important catalyst. We showed that the charge state of the cobalt center can be controlled by depositing the molecule on N-doped graphene. The oxidation state changes upon depositing on an N-pair in the doped graphene. This work was done in collaboration with the experimental group of Jerome Lagoute at the University of Paris-Diderot.
- M. Bouatou, S. Mondal, C. Chacon, F. Joucken, Y. Girard, V. Repain, A. Bellec, S. Rousset, S. Narasimhan, R. Sporken, Y. Dappe and J. Lagoute, Nano Letters 20, 6908 - 6913 (2020).
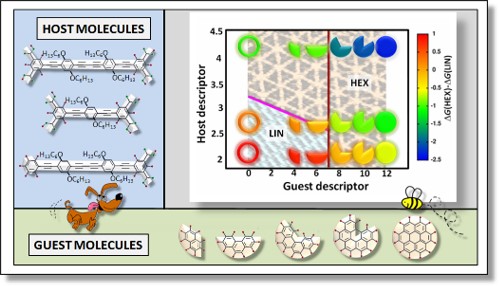
- Self Assembly of Organic Molecules on Surfaces We show that the geometry of the self-assembled structures formed when organic molecules are deposited on graphite can be captured by a simple descriptor that depends only on the geometric properties of the isolated molecules in the gas phase. This work was done in collaboration with the experimental group of K. George Thomas, IISER Thiruvananthapuram.
- P. Zalake, S. Ghosh, S. Narasimhan and K. G. Thomas, Chemistry of Materials, 29, 7170 - 7182 (2017).
Spin Crossover Molecules. Our collaborators (group of V. Repain at the University of Paris-Diderot) found that when a spin crossover molecule is deposited on Au(111) it forms an ordered lattice of high-spin and low-spin molecules. We show that this ordering arises from epitaxial strain imposed by the substrate.
C. Fourmental, S. Mondal, R. Banerjee, A. Bellec, Y. Garreau, A. Coati, C. Chacon, Y. Girard, J. Lagoute, S. Rousset, M.-L. Boillot, T. Mallah, C. Enachescu, C. Barreteau, Y. Dappe, A. Smogunov, S. Narasimhan and V. Repain, J Phys Chem Lett, 10, 4103 - 4109 (2019).
- Molecular rotors In collaboration with the experimental group of Anthoula Papageorgiou and Johannes Barth at the Technical University Munich, we have studied intriguing molecular rotors formed by depositing "BPP-COOH" molecules on Ag(111). We have shown that the rotational energy landscape can be explained by the making and breaking of host-guest hydrogen bonds. We also explain enantioselectivity in the system.
- D. Meier, A.K. Adak, P. Knecht, J. Reichert, S. Mondal, N. Suryadevara, S. K. Kuppusamy, K. Eguchi, M. K. Muntwiler, F. Allegretti, M. Ruben, J.V. Barth, S. Narasimhan and A. C. Papageorgiou, Angew. Chem. Int. Ed. 133, 27138 (2021).
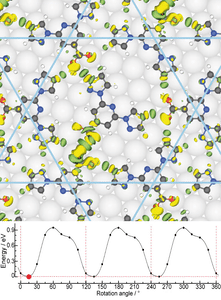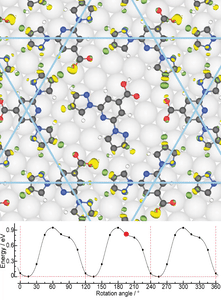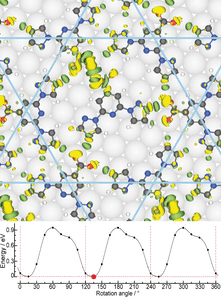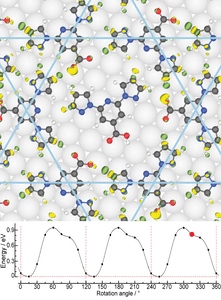
3) 2D MATERIALS
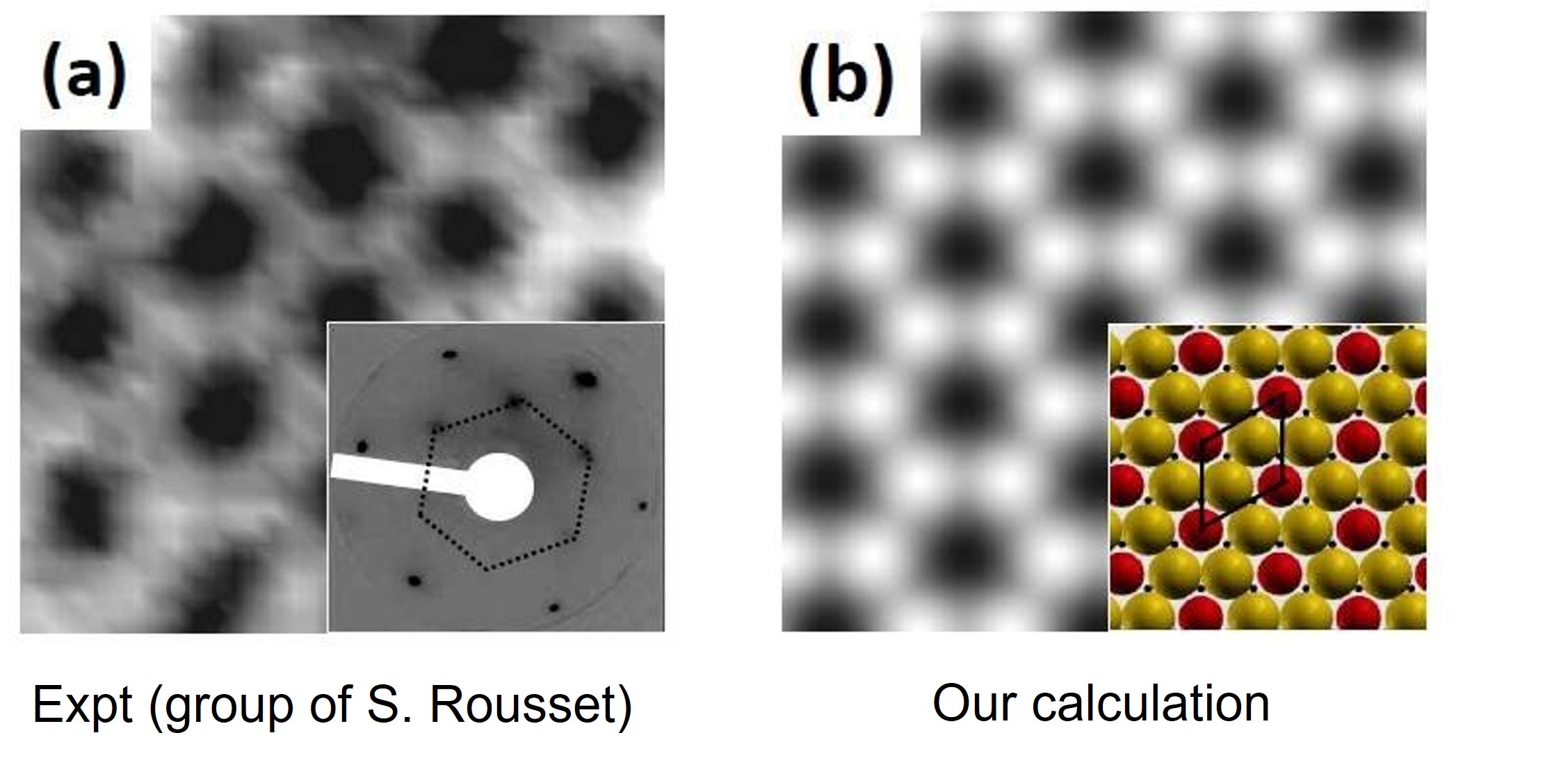
- Designing novel surface alloys: Gold and iron are essentially immiscible in the bulk. However, we showed that when deposited on a ruthenium surface, they form an atomically ordered surface alloy that is stabilized by magnetism.This alloy was successfully made by our experimental collaborators in the group of Sylvie Rousset at the University of Paris-Diderot.
- S. Mehendale, Y. Girard, V. Repain, C. Chacon, J. Lagoute, S. Rousset, M. Marathe and S. Narasimhan, Ordered Surface Alloy of Bulk-Immiscible Components Stabilized by Magnetism, Phys. Rev. Lett, 105, 056101 (2010).
- Synthesis of 2D materials: We have examined which metal substrates might be suitable for the synthesis of black or blue phosphorene, and have shown that which allotrope is favored can be tuned by varying the chemical potential of phosphorus.
- A.K. Adak, D. Sharma and S. Narasimhan, J. Phys. Cond. Matt. 34 084001 (2022).
- Defects in 2D Materials: We have studied defects in 2D materials such as twisted bilayer graphene, and (in collaboration with the experimental group of J. Lagoute at the University of Paris-Diderot) phosphorene. We have explained the source of puzzling features seen in STM images.
- Kanchan Ulman and Shobhana Narasimhan, Physical Review B 89, 245429 (2014).
4) Perovskites
- Delayed Fluorescence in Mn-doped Halide Perovskites: Our calculations show that vibrational coupling can help explain the delayed fluorescence observed when halide perovskites are doped with Mn. This work was done in collaboration with the experimental group of Ranjani Viswanatha at JNCASR.
-
P. Ramakrishnan, D. Acharya, P. Jain, K. Gahlot, A. Yadav, A. Camellini, M. Zavellani-Rossi, G. Cerullo, C. Narayana, S. Narasimhan and R. Viswanatha, ACS Energy Letters, 5, 353 - 359 (2020).
