Radiation Safety
Radiation Safety
The Radiation Safety Program provides guidance and oversight to users of radiation producing devices and radioactive material on campus and also for users of nonionizing radiation, including sources of ultraviolet, magnetic and micorwaves.
Radiation training in campus.
Even if you’ve attended radiation safety training at other institutions, you’re required to complete JNCASR safety training. If you’re not sure what training you should complete, contact the Radiology safety officer.
The type of training you need to complete depends on which form of radioactivity is used in your lab.
- If the radioactivity is present in a form that is easily dispersible, for example, liquids, powders and gases, it is an Open Source. Most biological and chemical applications use open sources.
- If the radioactivity is present in a form that is not easily dispersible due to its design characteristics, it is a Contained Source or Closed source
- Radiology safety in charges must ensure members of their labs are trained in the safe and proper practices for the procedures and materials used
What is Radiation
Radiation: energy in motion
Radioactivity: spontaneous emission of radiation from the nucleus of an unstable atom
Isotope: atoms with the same number of protons, but different number of neutrons
Radioisotope: unstable isotope of an element that decays or disintegrates spontaneously, emitting radiation. Approximately 5,000 natural and artificial radioisotopes have been identified.
Ionizing Radiation
- Occurs from the addition or removal of electrons from neutral atoms
- Four main types of ionizing radiation
- α - Alpha
- β - Beta
- γ - Gamma (X-ray)
- n - Neutron
Alpha (α) radiation:
- is not very penetrating and can be shielded by a few centimetres of air or a sheet of paper;
- is a significant internal exposure hazard;
- but can be difficult to detect because of the low penetrating power.
Beta (β) radiation:
- is more penetrating than alpha but still can be shielded by thick plastics or thin sheets of metal;
- is an external eye and skin exposure hazard;
Gamma (γ) radiation:
- has no mass and no charge but can be extremely penetrating;
- must be shielded by heavy or massive material such as steel, lead, or concrete (or a combination);
- is both an external and internal exposure hazard;
Radioactivity
- Rate of Decay / Potential to Decay
- “Strength”
- Curie (Ci) - 1 gram of radium disintegrates
- 3.7 X 1010 disintegration/second (dps)
- Becquerel (Bq) = 1 disintegration/second (dps)
- 1 mCi = 37 MBq
Units
| Sr. No. | Quantity | Old Unit | SI Unit | Relationship |
|---|---|---|---|---|
| 1 | Radioactivity | Curie (Ci) | Bq (Becquerel) | 1 Bq = 2.7 × 10⁻¹¹ Ci |
| 2 | Exposure | Roentgen | C/Kg | 1 R = 2.58 × 10⁻⁴ C/Kg |
| 3 | Dose | rad | Gy | 1 Gy = 100 rads |
| 4 | Equivalent Dose | rem | Sv (Sievert) | 1 Sv = 100 rems |
| 5 | Effective Dose | rem | Sv (Sievert) | 1 Sv = 100 rems |
Radioactive Decay and Half-life (T½)
For example, Cobalt-60 has a half-life of 5.2 years. i.e. the activity initially present will halve over 5.2 years and halve again over a further 5.2 years.
i.e. after 10.4 years, the remaining activity will be ¼ of that originally present.
Note: The radiation output (the gamma radiation emitted by the 60 Co) also will fall as the radionuclide decays. To ensure the correct dose is delivered to the patient during radiotherapy, treatment times must be adjusted regularly for the decay of the source.
To Minimize Contamination Risks
- Adopt clean operating conditions
- Adopt good laboratory practices - do not eat, drink, smoke etc…
- Use protective gloves and clothing
As Low As Reasonably Achievable
- How?
- Time
- Distance
- Shielding
- Why?
- Minimize Dose
Less time = Less radiation exposure
- Use RAM only when necessary
- Dry runs (without radioactive material)
- Identify portions of the experiment that can be altered in order to decrease exposure times
- Shorten time when near RAM
- Obtaining higher doses in order to get an experiment done quicker is NOT “reasonable”!
Distance
- Effective & Easy
- Inverse Square Law
- Doubling distance from source, decreases dose by factor of four
- Tripling it decreases dose nine-fold
- More Distance = Less Radiation Exposure
- Tongs, Tweezers, Pipettes, Pliers.
Shielding
- Materials “absorb” radiation
- Proper shielding = Less Radiation Exposure
- Plexiglass vs. Lead
Radiation monitoring
- Area Monitoring Instruments
- Personal Monitoring Instruments
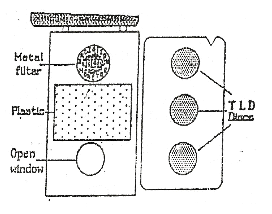
- TLD Badge: consists of three CaSO4: Dy discs embedded in a metallic framework and enclosed in a multi-filter cassette.
- The TLD badge can be used for monitoring doses due to beta, gamma and x-rays.
- Different filters provided in the badge enable to distinguish the doses due to different types of radiation.
- The TLD badge can cover a wide range of doses from 50µSv to 10 Sv.
- It is a mandatory requirement that all radiation workers in the country shall be enrolled in the Personnel Monitoring Services
GM Counter
- Operate at voltages above proportional detectors
- Each primary ionization
- produces a complete avalanche of ions throughout the detector volume called as Townsend Avalanche
- Avalanche continues until maximum number of ion pairs are produced, avalanche may be propagated by photoelectrons
- No proportional relationship between energy of incident radiation and number of ionizations detected
- Quenching is used
- Not useful in high radiation field or to measure pulsed radiation.

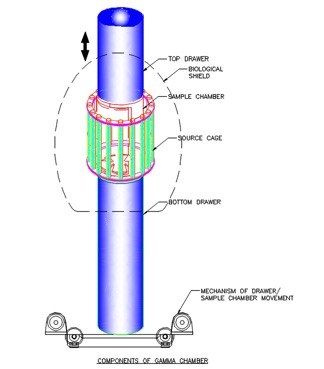
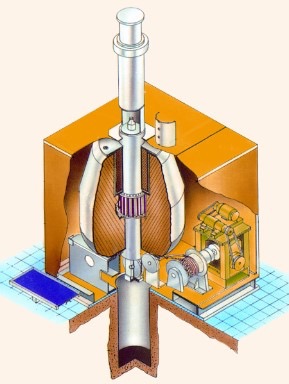
Gamma Irradiation Facility in campus
- Self - shielded Research Irradiators
- Irradiation volumes- 5000cc &1200cc respectively
- Radiation source – Co-60
- Source Capacity - 14000Ci & 5000Ci
- Central dose rate : 9 kGy /hr for both
- PLC control system
- Weight of the unit - 5.6 & 3.0 tons approx.
- Conforms to ANSI - N433.1-1977 irradiator code
- Type B (U) package approved by AERB, India.
- Space requirement: Room of size 4m X 4m X 4m (ht) approx.
- Units in use - 45 nos including 11 nos Abroad since its launch in 1997.
Applications
- Effects of radiation on materials, Radiation Chemistry, Preservation of Tissue Grafts, Food Preservation studies, Mutation Breeding, Material modification and radiobiology etc.
Radioactivity disposal procedure
- Disposal of short-lived solid radioactive waste
- Waste containing radioactive material with a half-life of 90 days or less is
- considered short-lived waste and keep all isotopes separate
- Dry waste consists of paper, gloves, plastic vials, tips, troughs and 96- well plates.
- Dry waste is collected in Perspex box that has been lined with a plastic
- bag, which is kept in the fume hood.
- Once the bag is full, the mouth of the bag is tied and the counts are monitored with the Radiation monitor. The counts and the date of monitoring the counts are written on the radioisotope label and stuck on the bag. \
- The bag is then stored in the radioisotope storage room for 10 half-lives after which it is disposed off as normal waste.
- Remove or obliterate all radioactive labels prior to disposal
- Survey with appropriate detector and confirm indistinguishable from background
- Maintain a record of waste generated and disposed.
General contamination procedure
- Use adsorbent paper on wet spill or wet absorbent paper on dry spill
- Repetitively swab the area inwards towards the center of the spill
- Place contaminated paper in a plastic bag or container
- Monitor the area
- Repeat all procedures until the exposure rate is below given limits
- If the decontamination is not successful, mark the contaminated area and classify the room as a controlled area (if not already done) until the contamination is completely removed.
PPE
- Lab coat
- Eye protection
- Closed toe shoes
- Personnel monitoring
- Gloves

For more assistance or in case of an emergency, please call the Radiology Safety Officer (RSO)080 2208 2710 immediately.
- Back to previous page
- |
-
Page last updated date:17-04-2025 12:17 PM























